
CEO Landon promotes BioBridge Global’s capabilities at Invest SA
BioBridge Global CEO Martin Landon spoke on a panel during the Invest San Antonio 2023-2024 Conference on Thursday, noting the organization’s growing capabilities.

BioBridge Global CEO Martin Landon spoke on a panel during the Invest San Antonio 2023-2024 Conference on Thursday, noting the organization’s growing capabilities.

South Texas Blood & Tissue leaders were featured in a webinar on an innovative program to collect whole blood-derived platelets. Audra Taylor (VP, Blood Operations)

BioBridge Global celebrated another major advanced therapies milestone March 1, opening its first stand-alone Cell Therapy Testing Laboratory.

The University of Texas at San Antonio and GenCure, a subsidiary of the San Antonio nonprofit BioBridge Global, have signed a master services agreement to collaborate on the development of cellular therapy products, services, and testing.

BioBridge Global is proud to announce that 12 abstract submissions were accepted to present as research posters during the AABB 2023 Annual Meeting taking place Oct. 14-17 in Nashville, TN.

A collation of Texas cities, including San Antonio, has landed one of three national hubs from the new federal Advanced Research Projects Agency for Health, an agency expected to send billions on healthcare research in the coming years.
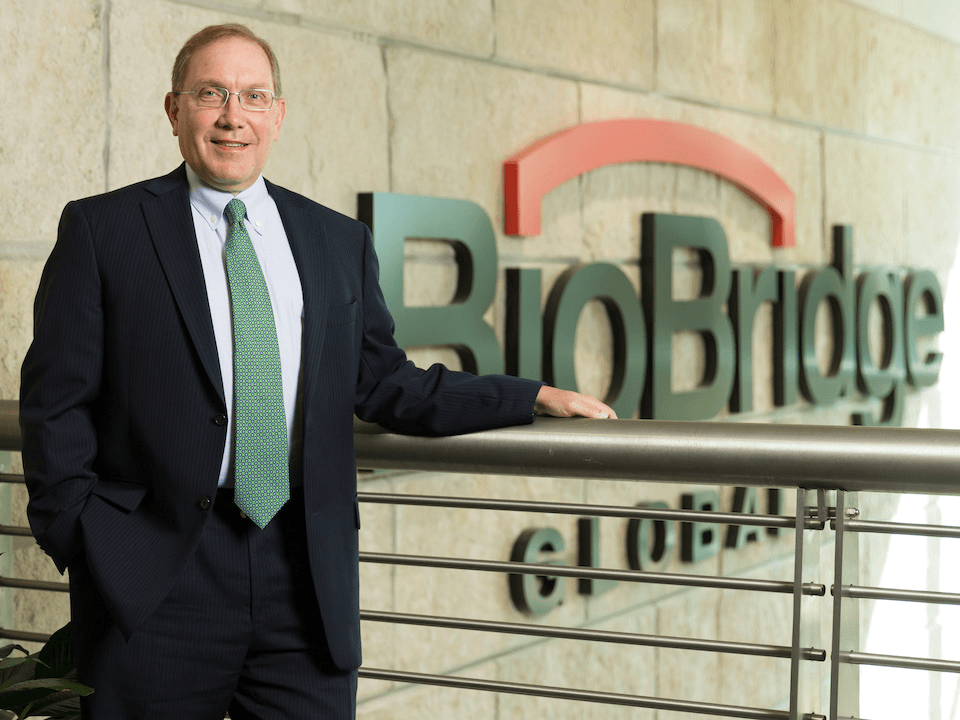
BioBridge Global CEO Marty Landon recently sat down with editors from Invest: San Antonio magazine for a Q and A on how the organization is meeting the demand for local healthcare and the growing advanced therapies market in today’s economic climate.
MasterControl has published a case study of its experience with BioBridge Global to demonstrate the effectiveness and abilities of its quality control and manufacturing solutions software in the advanced therapies industry.

South Texas Blood & Tissue, a subsidiary of San Antonio nonprofit BioBridge Global, is part of the Be The Match BioTherapies Cord Blood Bank Alliance that will provide research grade and cGMP umbilical cord blood units as a starting material for researchers and therapeutic developers.

South Texas Blood & Tissue has been presented the 2023 Partnership Recognition Award by the Texas Division of Emergency Management.

San Antonio and its surrounding areas are ripe for investment and opportunity, according to several city business leaders who presented at the Invest: San Antonio 2022-23 Leadership Summit.

South Texas Blood & Tissue’s Executive Director of Blood Operations, Audra Taylor, presented on the impact of the Heroes in Arms program to supply emergency vehicles and trauma centers with whole blood units in an online seminar during Blood Advocacy Week.